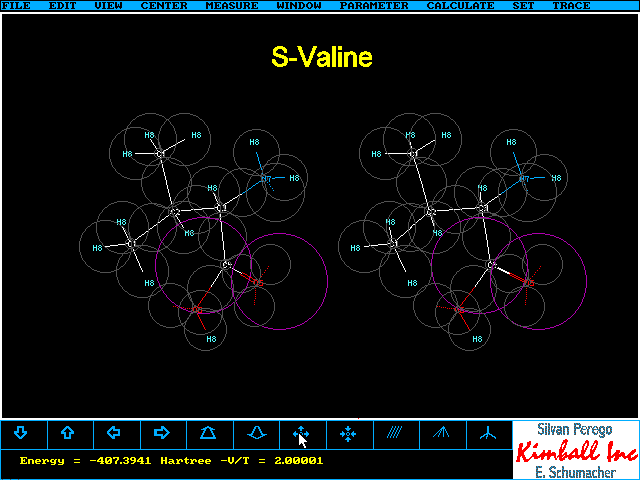
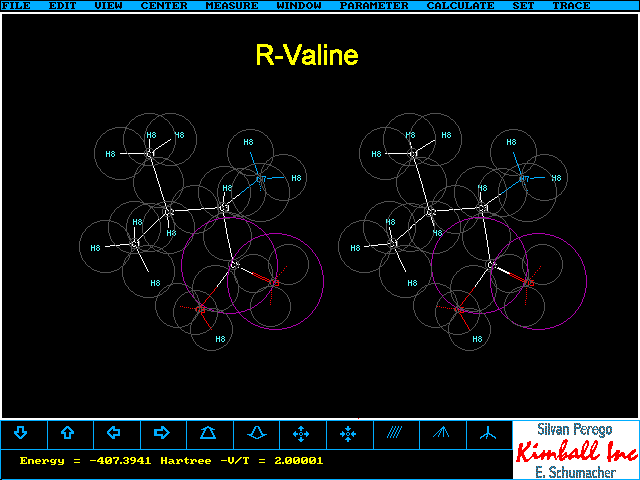
S- and R-Valine
These molecules can be built very easily by Kimball.exe.
If you just assemble the nodes without regard to stereochemistry you might
get either the S- or the R-enantiomer. Optimize the structure to obtain
the energy minimum. Now you don't have to repeat construction of the molecule
and optimization if this is not what you wanted to produce. A special trick
of Kimball.exe allows you simply to switch to the other eantiomer by mirroring
the molecular skeleton at the plane of projection:
 click this symbol (repeatedly) with the right mouse button: The stereo effect
of the projected structure is enhanced.
click this symbol (repeatedly) with the right mouse button: The stereo effect
of the projected structure is enhanced.
 click this symbol with the right mouse button to reduce the stereo effect.
If you continue clicking, the molecule passes through the projection plane
with zero extension in 3D-space and emerges as its mirror image. With the
symbol above you can reverse this process.
click this symbol with the right mouse button to reduce the stereo effect.
If you continue clicking, the molecule passes through the projection plane
with zero extension in 3D-space and emerges as its mirror image. With the
symbol above you can reverse this process.
With these two command buttons you may thus switch
from one to the other chirality and back as often as you wish.
Depending on whether you are accustomed to view
the 3D image nearer or farer to your eye than the monitor screen you will
see the two following pictures with reversed, but opposite, chirality.
- They are looking like their label implies when you view the 3D image
by squinting: Start with your index finger at your nose looking towards
the screen. Try to focus on the index finger and move this slowly towards
the screen until the 3D image hovers on your fingertip. This stereoimage
matches the labels written in the following pictures. You
can easily confirm the chirality by applying CIP rules.
- The other stereoimage is generated when you look at the left picture
with the left eye and at the right picture with the right eye and try to
fuse the two, slightly more distant than the monitor screen. Just relax
and use a hand or a card board to separate the left and right images properly.
If you are more accustomed to look at this stereoimage you will find the two
chirality descriptors reversed.