CH4, NH3, H2O, and HF molecules
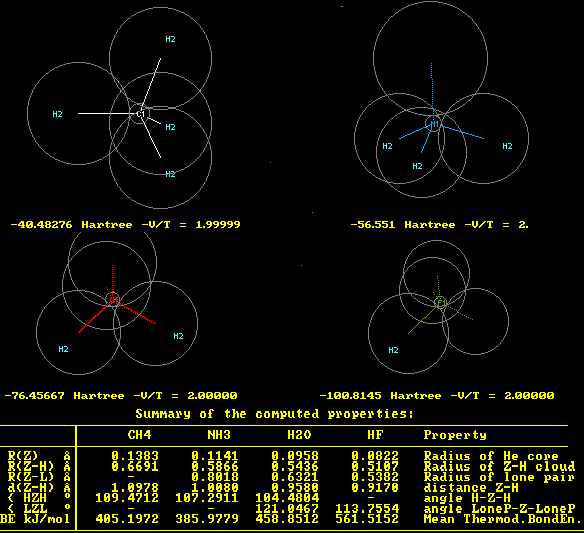
Since these molecules have been used for calibration of the set of parameters
it is not surprising, that they reproduce the known properties very closely.
It is, however, not trivial that Kimball's model is at all parametrizable
considering the drastic simplification inherent in its assumptions.
Even less trivial is the fact, that the parameters extracted from these
four structures also lead to good total and bond energies. And even more:
that they are transferable to the whole immense class of hydrocarbons,
carbon-nitrogen, carbon-oxygen, carbon-fluorine etc. compounds. Additional calibrations with C2H6 for the CC-bond and several others for
the CN-, CO-, CF-, NN-, NO-, NF-, OO-, OF-, C=C-bonds are necessary to do
so.-
The indices 1,2 at atom symbols are not atomic multipliers, just class labels,
e.g. CH4 has two atom classes: C1, H2, a.s.o., which the program assigns (can be hidden).
For another parametrization see LiH...HF.