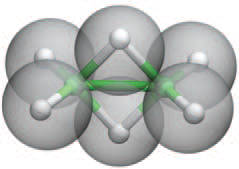
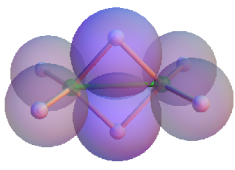
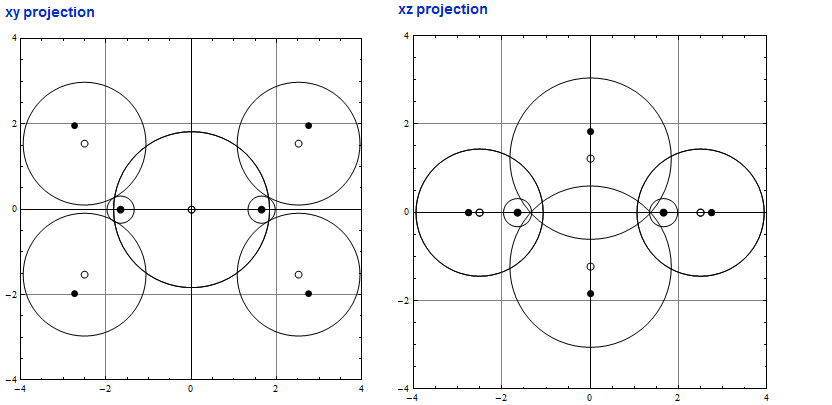
So here is my solution or its CDF and two projections of the computed model. The protonated "double bonds" must overlap! Shame to HAB! Otherwise no reasonable molecule is possible.
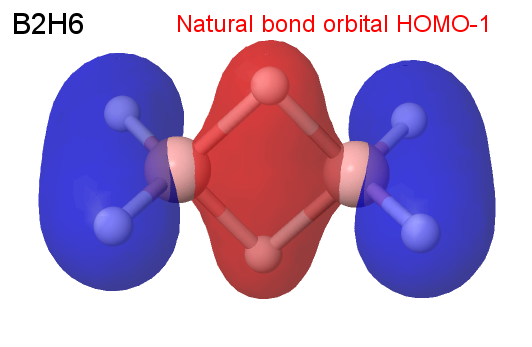
This is in accord with an MP2/6-31G(d) optimization. The NBO-7 = HOMO-1 clearly shows the accumulation of charge in the B-B axis:
or the same (Kohn-Sham) orbital from a CPMD 3.15.3 computation:
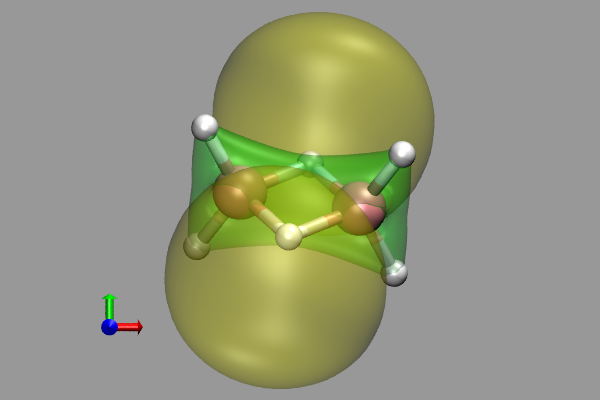
It is very interesting that the new approach of Su and GoddardIII (2009) with FSGO's and using their program eFF (J.Su & W.A.Goddard III, JCP 131,244501(2009), Fig. 13), left model, leads to a similar molecular construct as the Kimball result, right: