The protagonists H, H2+, H2, He, H-, H3+, H3: in preparation
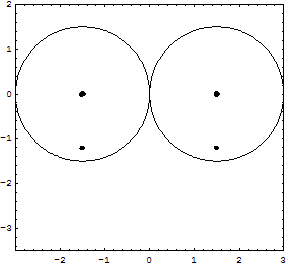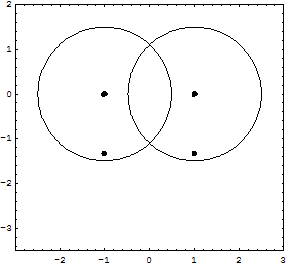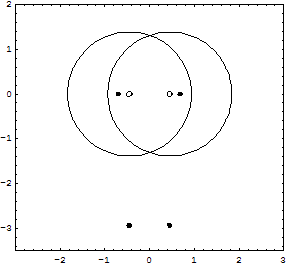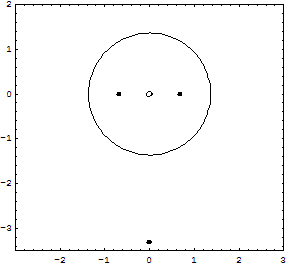
Details for the calculations are given in the Tutorial; original Kimball version, experimental numbers in parenthesis.H-Atom: Variable R, Parameter ZEkin = 9/8R2; Vne = -3Z/2R; Vee = 0; Vnn = 0 Etot = Ekin + Vne; Min[Etot(R)] = -Z2/2 Eh; R = 3/2Z ao (exact match of experiment) Extension with principle quantum number n produces the H-atom spectrum. H2+ molecular ion: Variables R, rEkin = 9/8R2; Vne = -3/2R +1/2*(r/R)2/R; Vnn = 1/2r; Etot = Ekin + Vne + Vnn; Min[Etot(R,r)] = -0.7276 (-0.6026) Eh; R = 1.2435 ao; 2r = 0.829 (1.058) Å H2 molecule: Variables R, rEkin = 2*9/8R; Vne = 2*2*(-3/2R + 1/2*(r/R)2/R); Vee = 6/5R; Vnn = 1/2r; Etot = Ekin + Vne + Vee + Vnn; Min[Etot(R,r)] = -1.12 = -1.21 (-1.1745) Eh; R = 1.5/1.1 ao; 2r = R = 0.7216 (0.7414) Å It is interesting, that Kimballs model gives one stable H2 molecule and an infinite series of metastable "precursors" until both protons are on the fringe of the other H-atom: At right the last metastable form is shown with about half the binding energy of the stable H2, and a H-H distance 3% larger (optical illusion makes it smaller!). This is an artifact of the homogeneous charge distribution of the model. Check the forces! The protons have to actually penetrate the other H-atom for the stable H2 on the left to be produced. This has equal H-H distance and cloud radius with H-clouds fused completely. See below for an animation.   He and He like ions (including H-): Variable R, Parameter ZEkin = 2*9/8R; Vne = Z*(-3/R); Vee = 6/5R; Etot = Ekin + Vne + Vee; Min[Etot(R)] = -(Z-0.4)2 Eh; R = 1.5/(Z-0.4) ao For He we get Etot = -2.56 (-2.91) Eh and for its ionization potential 0.56 (0.91) Eh H3+: Variables R, r; centered equilateral triangle formed spontaneously from dashed start structureEkin = 2*9/8R; Vne = -6*(3/2 - 1/2*(r/R)2)/R; Vee = 6/5R; Vnn = 3^(1/2)/r Etot = Ekin + Vne + Vee + Vnn; Min[Etot(R,r)] = -1.6631 (-1.3289) Eh; R = 1.1631 ao; r = 0.7687 ao; d(HH) = 1.3314 (1.70) ao; dashed circle is the H atom cloud for comparison, solid circle the contracted result for the field of three protons. Full computation in H3+gen.nb 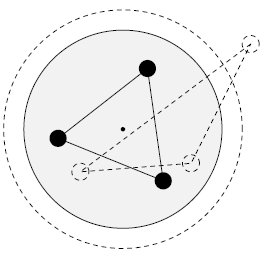 H3: Variables R1, R2, r; linear H-H-H formed spontaneously, Pauli exclusion obeyed; not stable against -> H2 + H predictedEkin = 9/8R12 + 9/4R22; other terms see in H3lina.nb, first computed by G.F.Neumark, thesis loc.cit. p.27(1951) Etot = Ekin + Vne + Vee + Vnn; Min[Etot(R1,R2,r)] = -1.7090 (not av.) Eh; R1 = 1.7254 ao; R2 = 1.3000 ao; d(HH) = 1.5352 (na) 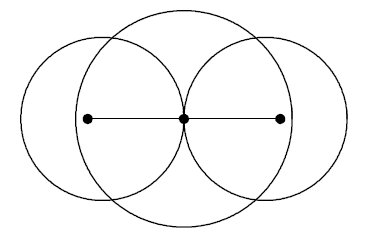 |
Weaknesses:
1) Electron-electron repulsion of pair in same cloud, spins different:
This produces a screening of the nuclear charge for each electron. Assuming equal density and distribution of both electrons over the cloud give a screening constant of
Hence, the error originates from neglecting correlation energy: The electrons of the pair do not independently move through the whole cloud (space). They prefer being as close to the nuclei as possible but simultaneously avoid each other as much as possible. There is no simple scheme to correct this gross error in Kimball's model nor in Hartree-Fock theory (see any text of QC)! In order to rationalize Slater's value of σ = 0.3 we try to model it:
a) e1 is mainly in an inner sphere with r1 while e2 is in an equal volume outer spherical shell R-r1 -> σ = 0.37
b) the average distance of e1 and e2 is <r> = R -> σ = 0.3333 (best try and better than 0.3 for higher shells)
c) each electron is predominantly near the center of mass of the other halfsphere
-> σ = 0.4444; even worse!
d) apply E. Wigner's correlation hole function: would destroy the simplicity of Kimball's model
Conclusion: we accept Slater's screening constant for 1s2 and determine others by parametrization with a good (correlation corrected) QC result. This applies to all electron pairs in Kimball calculations and is shown in the examples of the main page.
2) High bond energy, low distance in H2+, H2, H3+:
This is an effect of the inability of Kimballs ansatz to model the density cusps at the nuclei, partially relieved by FSGO, floating spherical gaussian orbitals, which destroy the simplicity of the K-model.
Conclusion: We can ± save the best case of a didactical introduction of chemical bonding with a small adjustment of the kinetic energy of the K-model.
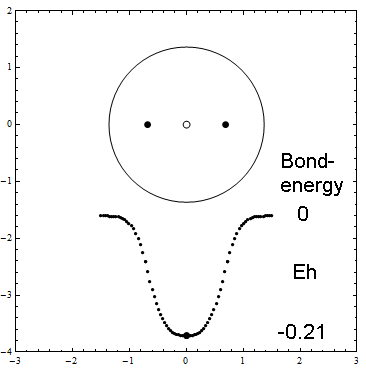
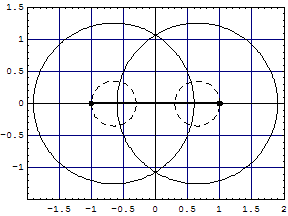
Here is an animation of the formation of H2 from two H atoms starting to penetrate. These are 41 frames of nonempirical, converged Kimball computations, see H2stat. The two lower points show the binding energy (the attractive part of the potential curve) as function of the distance of the H atoms: from far to touching, the atoms have zero interaction according to Kimballs model. With penetration the energy slowly falls, then rapidly reaches the stationary minimum (scale can be read in H2stat) when each proton enters the other cloud as well. Watch the small proton disks, which move within the open circles of a cloud center but detach from it when entering the other cloud. Pressing the protons together would produce the repulsive part of the potential curve. And make sure to observe the size change of the clouds when nearing the stationary H2 molecule.