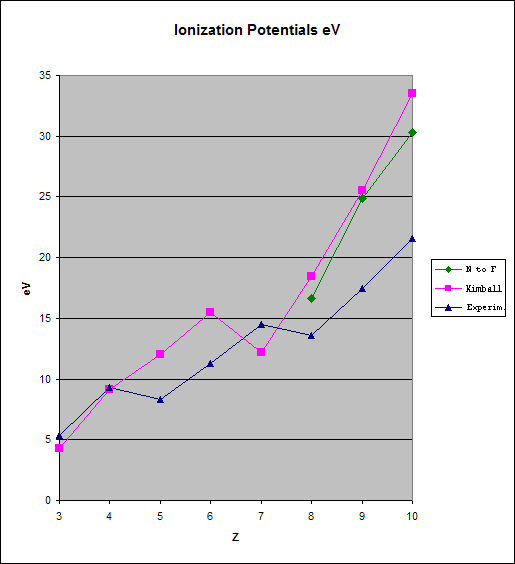
For these the Aufbauprinciple requests and explains the blue IP sequence. For the pink Kimball values this is definitely incorrect. Ok. But, what do you try to understand? Lewis' and Kimballs chemical atoms are meant to be used in a chemical context, which is never spherically symmetric. For this the spherical eigenstates of an isolated atom are inadequate and don't apply. The four-"valent" Lewis/Kimball C-atom has been replaced by the physicists spherical 2s2p2 atom. This caused a problem for every chemistry teacher: Why does the C-atom with two half filled orbitals form four and not only two electron pair bonds? The problem does not exist if the cart is not put before the horse. Symmetry comes before the eigenstates because the symmetry of the external perturbing field creates the correct eigenstates and not the other way round: If the isolated, spherical C-atom with 2s2p2 3P senses ligands