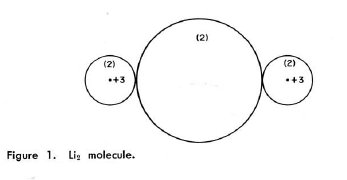
|
G.E. Kimball & E.M. Loebl, J.Chem.Educ. 36(1959)233 discuss (on page 235) the molecular orbital treatment of Li2 in the form of an LCAO-MO approximation. Summary: The linear combination of 1s, 2s (and 2p?) AO's undergoes drastic changes by antisymmetrization, leading to exchange and Coulomb interaction terms. The energy partition between these two categories is arbitrary and leaves the total energy invariant. By proper choice of components of the wavefunction one can minimize the exchange and maximize the Coulomb contributions. What then survives of the originally used linear combination of orbitals after the minimization of total energy resembles the electronic density distribution in Fig. 1 on p. 235, reproduced here. It shows - without scale given - the contours of three non-overlapping spheres in a plane containing the Li nuclei, and is a template for a Kimball model calculation (outlined but not realized in the paper). |
 |
|
I do not question the authors' arguments, but compute Li2 as Kimball model with variations. Li-Li is the first non hydrogenic bond of the periodic table and thus warrants closer inspection, because the bonding cloud has no nucleus embedded. AFAIK no Kimball model computation of Li2 has been published. But LiH was treated by L.M.Kleiss(1952, loc.cit. p. 10-12,37). The above figure seems to be a natural continu-ation, removing two H atoms from two LiH and combining the two half occupied Li(2s) clouds. |
|
The external Li2 after Kimball-Loebl (above 4) and the internal (above 5) are superimposed on the Li2 total density contour plot in the famous 'Pictorial Studies of Molecules' by Arnold C. Wahl, ANL-7076, 1965. Scale: The innermost contour corresponds to 1 electron per bohr^3. The next 14 contours are always 1/2 of the density of the contour above: 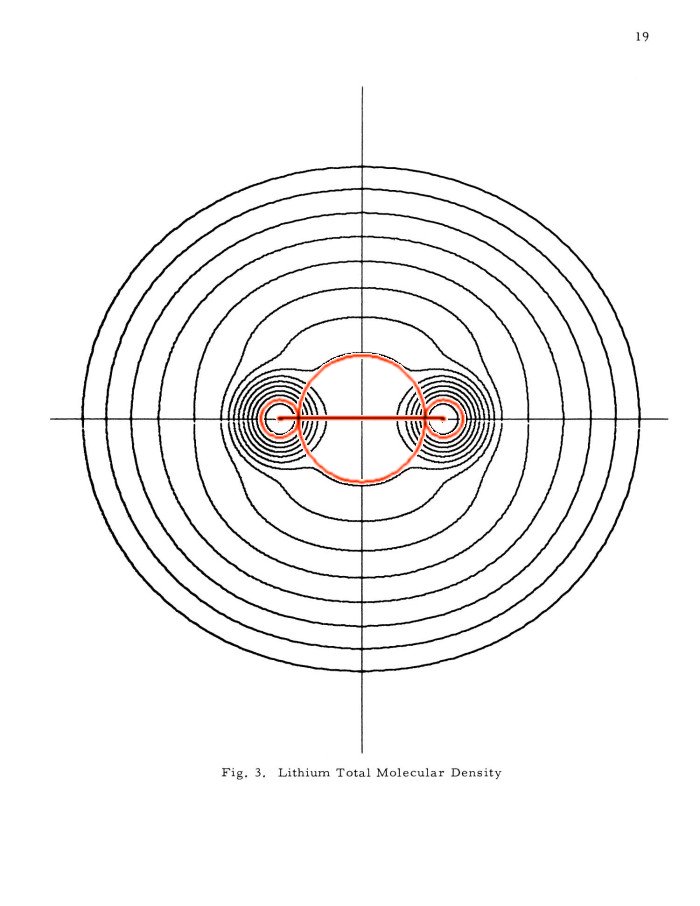
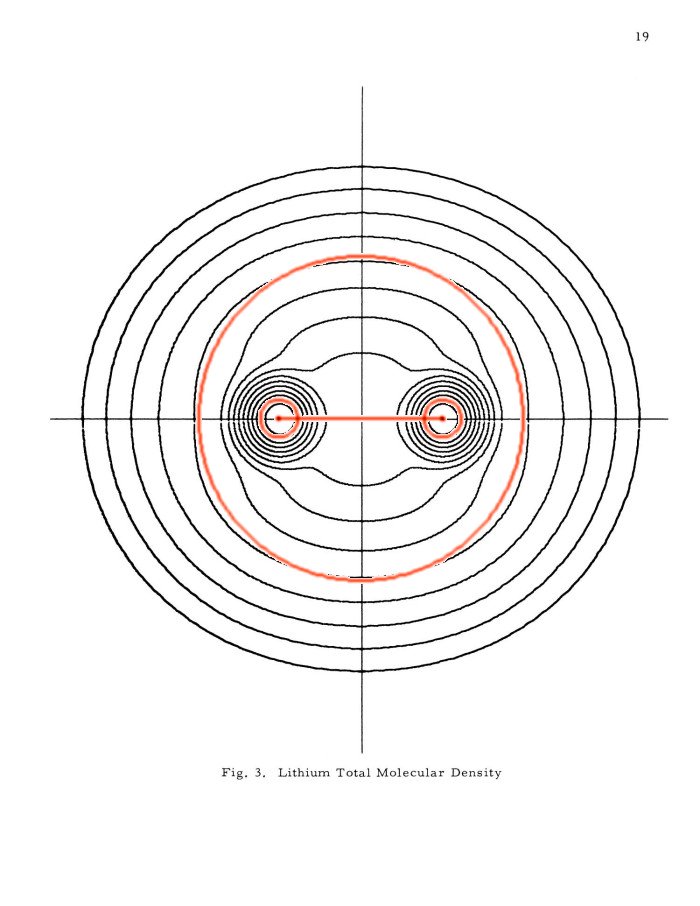
While the Kimball-Loebl model, left, needs more parametrization to resemble the experimental molecule, the internal model, right, requires only small adjustments of the original Kimball parameters. But, it is necessary to use the bonding cloud with a 2s kinetic parameter (H.R. Westerman, 1952, loc.cit. main page, has first treated free electron clouds with higher quantum numbers, ns- and np-orbitals, n <= 2). Which one shows the more natural representation of the Li-Li bond ? It depends on what you try to understand. The right model is a pleasing development of what is known about the H2 molecule, with very little exchange correction, because overlap of the valence electrons with the 1s2-cores is so small. But, Li2 is far from similar to H2! It is a strong tripole (a linear quadrupole), cannot be isolated but condenses to a metallic extended structure with broken-up electron pair if brought in contact with others. In the metal there are 8 or more clouds around each Li core producing a terrible conundrum of Pauli exclusion holes and huge exchange corrections with the model on the right. If this gets disentangled (by antisymmetrization) you end up much nearer to the left model, a Li+ in a sea of half filled external clouds connecting the whole structure and giving it metallic conductivity. Electrons can jump around the halfempty "cloud (anion-sub-)lattice", almost freely, a "Lithium-electride salt". Solid state physicists talk about a half filled valence band, looking at the energylevels, but mean the same thing.
|