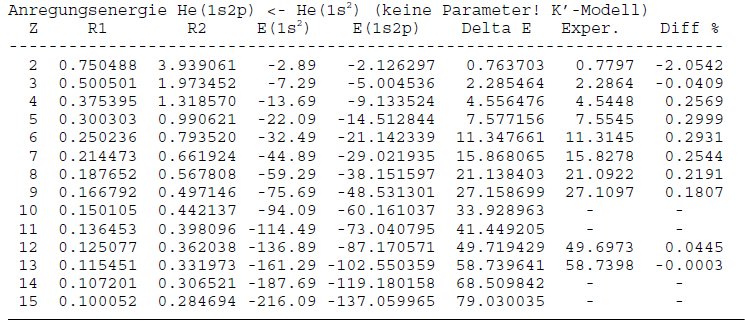
Intermolecular and Van der Waals interactions, Dipole Moments, Vibrations
Stable Kimball molecules like BH3, AlH3, AlCl3 dimerize in the way we know from experiment. The dimers are more stable than twice the energy of monomers. This has been shown by L.M. Kleiss, diss.loc.cit. Since Kimball's clouds are assumed not to penetrate between different monomers, van der Waals interaction happens exclusively through dipole-dipole (perhaps dipole-induced dipole) interaction. Kleiss has shown, that the special dipole interaction connected to H-bond formation can be computed between NH3, H2O, HF molecules and any mixture of these. CH4 pairs do not show any interaction when touching. The numeric values are not good, since Kimballs model overestimates dipole moments by about a factor of 2, due to the unphysical charge distribution which does not model the density cusps near nuclei. For the same reason, vibrational frequencies are too high and energy |
barriers for internal rotation, e.g. in ethane, too low but qualitatively correct. You can select the computation of dipole moments and vibrational frequencies/force constants in Perego's Kimball.exe and check these terse statements.
Positronium, Myonium and friends
By simply adding reduced mass one can include the motion of nuclei and their kinetic zero point energy for computing the ground state of molecules. Changing the mass of the electron allows to model molecules with heavier electrons. A special case is positronium, where the nucleus of the H atom is replaced by a positive electron. Since this has the same kinetic zero point energy as the electron with the same +/- interaction energy as for the H atom, the ground state energy is correctly determined as one half of that of the H atom, -1/4 Eh. Similarly all other short lived aggregates of matter-antimatter particles can be computed with Kimballs model (E.Schumacher, unpublished, 1961).
| |