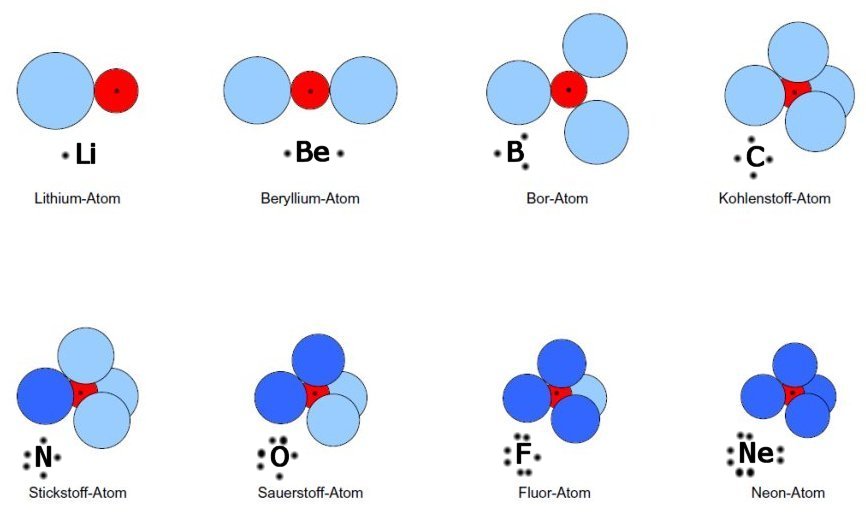
With and without rabbit ears
I'm not sure whether G.E. Kimball would have liked this qualitative picture and know that some theoretical chemists give a deadly verdict like "trash" to it. However, although you will not find this exact picture anywhere in the Columbia theses of Kimball's doctorands, they are there and used, e.g. for the hydrides Li to HF (L.M. Kleiss, loc. cit.) and reactions of C with H. Furthermore, G.E. Kimball and J.G.Trulio, JCP 28,493(1958) have presented the "Quantum Mechanics of H3" and given the proof that all molecular structures can be described accurately with a large -> infinite number of H(1s)-functions. That is essentially, what the above picture of (a still small number!) of non-overlapping spherical functions i of variable R(i) shows, R(i) depending on interaction with the Coulomb fields of the other particles nearby and their quantum chemical zero point kinetic energy. These "rabbit-ear" atoms are just a bit more elaborate than G.N.Lewis' single and double dots with which he decorated atom symbols to express their "bonding number". This has been accepted by chemists since the thirties of last century. Lewis tried | in vain to attach quantitative significance in the sense of energy values to this electron pair bonding scheme (in 1916 QM was still 10 years away). G.E. Kimball, however, has succeeded to attach a quantitative aspect to a blown-up spherical Lewis-dot, avoiding the big machinery of Quantum Chemistry, many chemists do not properly understand, anyway! Lewis' dashes and dots, even tapered to render them 3D aware, are a big part of the symbolic language of organic chemists. It is loaded with a wealth of empirical data not directly visible with the formulae. An analogy would be the score of a symphony which conveys pitch and duration of notes but presupposes, that every instrumentalist has learned over many years how to interpret the composers intentions behind the abstract notation. Most theoretical chemists have never learned nor understood the practical chemists' language. Hence, their verdict in regard to simple models is inadequate, incompetent and may safely be ignored. Organic chemists get results with this symbolic art in minutes without using a computer and often in cases where todays quantum chemists dare not even try.
|